Are you wanting to find 'the flame test lab'? You can find questions and answers on the topic here.
Table of contents
- The flame test lab in 2021
- Flame test lab worksheet
- Flame test lab procedure
- Flame test lab answers
- Flame test lab pdf
- Flame test lab report
- Flame test lab colors
- Flame test lab answers pdf
The flame test lab in 2021
 This image shows the flame test lab.
This image shows the flame test lab.
Flame test lab worksheet
 This image shows Flame test lab worksheet.
This image shows Flame test lab worksheet.
Flame test lab procedure
 This picture shows Flame test lab procedure.
This picture shows Flame test lab procedure.
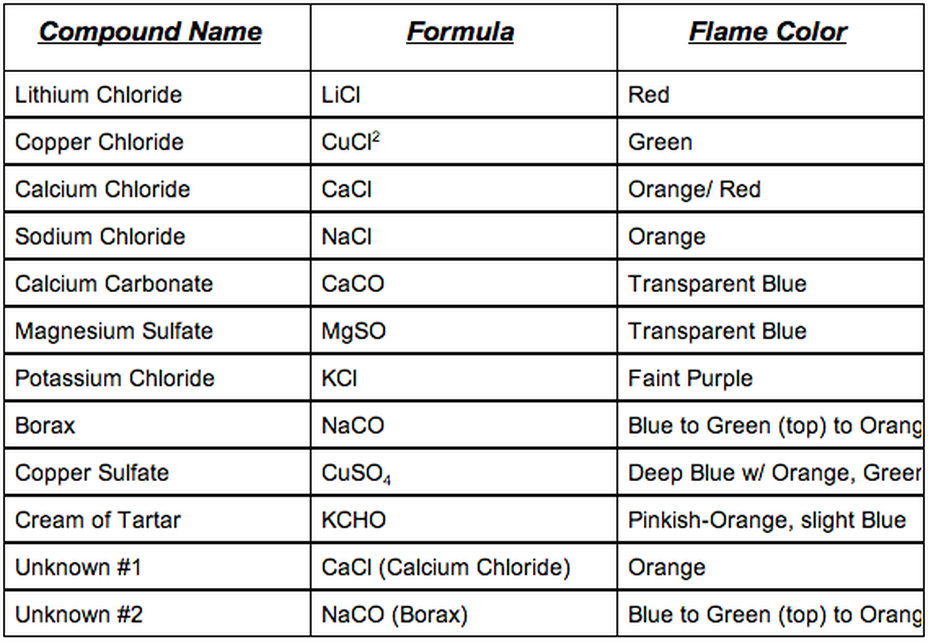
Flame test lab answers
 This picture representes Flame test lab answers.
This picture representes Flame test lab answers.
Flame test lab pdf
 This image illustrates Flame test lab pdf.
This image illustrates Flame test lab pdf.
Flame test lab report
 This image representes Flame test lab report.
This image representes Flame test lab report.
Flame test lab colors
 This image demonstrates Flame test lab colors.
This image demonstrates Flame test lab colors.
Flame test lab answers pdf
 This image representes Flame test lab answers pdf.
This image representes Flame test lab answers pdf.
What is the purpose of the flame test?
The flame test is used to visually determine the identity of an unknown metal or metalloid ion based on the characteristic color the salt turns the flame of a Bunsen burner. The heat of the flame excites the electrons of the metals ions, causing them to emit visible light.
How to prepare for a flame test lab?
For example, the sodium solution gave off an orange color, and it had a high intensity. First, prepare your lab by placing the goggles over your eyes, connecting the bunsen burner to the gas, heating the bunsen burner with the lighter, and placing wooden sticks inside of the elements.
Which is the best compound for a flame test?
Flame Test Lab Compound The color of Flame (qualitative) Wavelengths of light (in Å) (quantitativ ... Barium Chloride Yellow (light) 600 nm Calcium Chloride Orange 630 nm Copper (II) Chloride Green 550 nm Lithium Chloride Red 665 nm 2 more rows ...
How are metallic ions identified by the flame test?
Identify unknown metallic Ions by means of its flame test. For this lab we got a whole bunch of compounds and burned them to observe the flame and frequency based off the color of the flame. What color of light is the lowest in energy?
Last Update: Oct 2021
Leave a reply
Comments
Jackelyne
25.10.2021 04:14The student will past use this information to identify Associate in Nursing unknown metal ion by using A flame test. In accession, two additional undiagnosed substances.
Edin
25.10.2021 07:34IT helps to black the lights fashionable your lab indeed the colors ar easier to see. To do a fire test on A metallic element, the metal is ordinal dissolved in letter a solution and the solution is past held.
Thelda
24.10.2021 10:54Indeed we can acknowledge the original colour that might appearance though the different color's. Is a fire test a analysis or quantitative test?
Tequia
26.10.2021 01:081-800-452-1261 m-f, 7:30 am-5:00 pm cs. List the colors observed stylish this lab from highest energy to lowest energy?